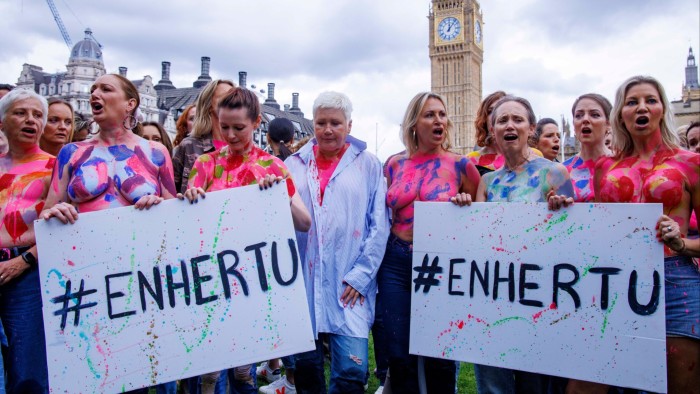Unlock the Editor’s Digest for free
Roula Khalaf, Editor of the FT, selects her favourite stories in this weekly newsletter.
England’s drug approval body has resisted pressure from pharmaceutical companies to loosen its process for recommending drugs and is pressing instead for the NHS to speed up the rollout of approved medicines.
Ministers have been putting pressure on the National Institute for Health and Care Excellence (Nice) to show how it is increasing productivity and contributing to economic growth, at a time when pharmaceutical groups are railing against recent high-profile rejections of new medicines.
Enhertu, a novel breast cancer drug developed by AstraZeneca and Daiichi Sankyo, was rejected by Nice last year on the grounds that it did not provide value for money, even though it was approved by Scotland and 22 other European countries.
As part of efforts to prove its contribution to growth, Nice, an arm’s-length body of the Department of Health and Social Care, has started producing financial estimates of the economic benefits of efficient drug rollout, it told the Financial Times.
The estimates are in part aimed at persuading ministers and the NHS to buy more of the medicines it recommends as value for money and increase supply.
For example, the institute has compiled evidence suggesting that the rollout of an implant for musculoskeletal joint pain could enable 13,296 people to move from inactivity into employment, boosting the economy by £500mn — while the rollout would cost the NHS £277mn.
Nice is also in talks with ministers about ways in which novel medicines could be administered outside of GP surgeries, including by online providers and pharmacies — a shake-up set to form part of the government’s 10-year plan for the health service, according to people briefed on the discussions.
Following Enhertu’s rejection, health ministers consulted Nice about whether its formula for determining drug approvals needed to be amended, said people familiar with the matter.
Pharmaceutical groups have argued for changes to how the formula is applied, including metrics such as the quality-adjusted life year, which is used to measure how many healthy years a drug buys, and the “severity factor”, used to assess the impact of diseases.
However, last year an in-house review of the effectiveness of Nice’s formula concluded that no changes were needed.
“We are not going to start paying more for medicines — that would simply blow the government’s medicines budget and ministers understand that,” said a person briefed on Nice’s thinking. “But we can buy greater quantities of medicines. This is about creating an argument to get the NHS to buy more.”
Even when Nice recommends that drugs are value for money, NHS trusts can still fail to adopt them.
Most prominently, the body approved the weight loss and diabetes drug tirzepatide, made by US group Eli Lilly and sold as Mounjaro, but the NHS said it needed to slow the rollout to be able to afford the medicine for the millions of obese patients who would qualify.
“The assumption is that it needs to be GPs doing it, but why? We can’t repeat a system where it takes 12 years to roll out a good medicine,” said a person close to discussions between ministers and Nice, referring to the expected timeline for the rollout of tirzepatide.
The health department declined to comment.